Enantioselective Synthesis
When a new stereo centre is created two stereo isomers(enantiomers) may be formed. If one of the enantiomer is formed in significantly higher proportions theprocess is termed enantioselective synthesis.When a stereo centre is already present and a new one is created, there are two stereo isomers (diastereomers) possible. If one of them is formed in significantly greater proportions the process is termed diastereoselective synthesis. In some reactions there is a possibility of formation of several stereo isomers, in such cases the preferential formation of one over the other is diastereoselectivity.
Epoxidation of allyl alcohols (Sharpless epoxidation)
- When an alkene is treated with a peracid or a hydroperoxide an epoxide (oxirane) is formed.
- Allylic alcohols give mainly one enatiomer when epoxidised.
Substrate: Allyl alcohol.
- Reagent(oxidant): t-butylhydroperoxide.
- Catalyst: Ti-tetra isopropoxide. Ti(OCHMe2)4.
- The tartarate is responsible for the stereo selection. (2R,3R)-diethyl tartarate (+) DET gives mainly one enantiomer while .(2S,3S)-tartarate(-)DET gives the other enantiomer as a major product as shown in the diagram.
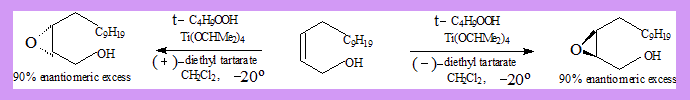
It is believed that oxygen is transferred from hydroperoxide to the alkene in a complex in which the configuration of the ester group determines whether oxygen is delivered from above or below the plane of the alkene.
The catalyst binds the hydroperoxide, allylic alcohol group, and the asymmetric tartrate ligand via oxygen atoms in the transition state.
Hydroboration-Oxidation
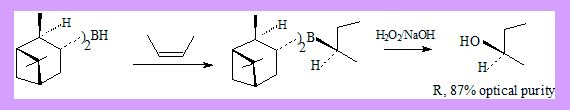
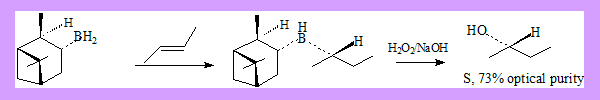
Hydrobortion and oxidation is a well known method of preparing alcohols from alkenes. The alcohol formed is an anti-markonikov product. Conditions: Hydroboration: BH3 / THF Oxidation: H2O2 / NaOH If the alcohol formed has a new stereo centre the product is a racemic mixture. Hydroboration of alkenes by using chiral alkyl boranes followed by oxidation leads to asymmetric synthesis of secondary alcohols. Two such alkyl boranes are:

These reagents can be readily prepared by reaction of borane with (+) or (-)-α-pinene under appropriate conditions. Many more derivatives of the reagent have been used leading to better results.
- Z-2-Butene with (-)disopinocampheylborane followed by oxidation with alkaline H2O2 gives R-2-Butanol with 87% optical purity.
- With E-disubstituted alkenes best results have been obtained with reagent IpcBH2. The success of the reaction depends on the bulkiness of the alkyl groups on the double bonds. (E)-2-Butene ? (S)-2-Butanol 73% optical purity.
- (E)-di-t-butylethene results in corresponding alcohol with 92% optical purity.